
Anesthetics
Research on anesthetics at the ICMS focuses on the mechanisms by which volatile and intravenous general anesthetics modulate ion channels.
Structure of target receptors
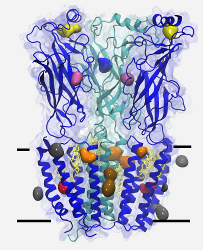
nAChR: We have proposed that cholesterol (which is required for nicotinic acetylcholine receptor function) is deeply embedded in the transmembrane portion of the structure, and developed a model of a cholesterol-nAChR complex suitable for computational use. This model has been evaluated in simulations ranging up to 500 ns in length, and shown to significantly stabilize the receptor with respect to the control system.
K2P: We have built a model for the anesthetic-sensitive tandem pore domain potassium channel TREK-1, including the cytoplasmic domain. Although the cytoplasmic domain is initially constructed as a dimer extending into the cytoplasmic region, multiple simulations demonstrate that it docks as two monomers to the intracellular leaflet of charged lipid bilayers.
Anesthetic Force-field Development
Recently, CHARMM-compatible models for isoflurane and propofol have been developed within our group. Isoflurane parameters are available here, while propofol parameters are available upon request. Both models reproduce correct solvation free energies in TIP3P solvent.
Flooding of receptors by anesthetic
In this method, a high concentration of the anesthetic is placed in the aqueous phase of the simulation and allowed to partition into membrane and protein binding sites until a clinical concentration in water is reached. We have very recently provided detailed visual images of binding sites for isoflurane on nAChR and the homologous prokaryotic channel GLIC, demonstrating that the primary method of inhibition by isoflurane in these two channels is likely via pore-block. We are presently investigating the effect of isoflurane bound to potentially allosteric sites on nAChR dynamics.
References:
- A. F. Barber, V. Carnevale, S. J. Raju, C. Amaral, W. Treptow, M. L. Klein, BBA-Biomembranes, 1818, 2120-2125 (2012). (Link)
- D. N. LeBard, J. Henin, R. G. Eckenhoff, M. L. Klein, G. Brannigan, PLOS Comput. Biol., 8 (5), e1002532 (2012). (Link)
- G. Brannigan, D. N. LeBard, J. Hénin, R. G. Eckenhoff, M. L. Klein, Proc. Natl. Acad. Sci. USA , 107(32), 14122-14127 (2010). (Link)
- W. Treptow, M. L. Klein, J. Am. Chem. Soc., 132, 8145-8151 (2010). (Link)
- J. Hénin, G. Brannigan, W. Dailey, R. Eckenhoff, M. L. Klein, J. Phys. Chem. B, 114, 604-612 (2010). (Link)
- Satyavani Vemparala, Carmen Domene, Michael L. Klein, Accounts of Chemical Research 43, 103-110 (2010) (Link).
- L. Vedula, G. Brannigan, N. Economou, J. Xi, M. Hall, R. Liu, M. Rossi, W. Dailey, K. Grasty, M. L. Klein, R. Eckenhoff, P. Loll, J. Biol. Chem. 284, 24176-24184, (2009). (Link)
- C. A. Butts, J. Xi, G. Brannigan, A. A. Saad, S. P. Venkatachalan, R. A. Pearce, M. L. Klein, R. G. Eckenhoff, I. J. Dmochowski, Proc. Natl. Acad. Sci. USA 106, 6501-6506 (2009), (Link)
- G. Brannigan, J. Hénin, R. Law, R. Eckenhoff, M. L. Klein, Proc. Natl. Acad. Sci. USA 105, 14418-14423 (2008). (Link)
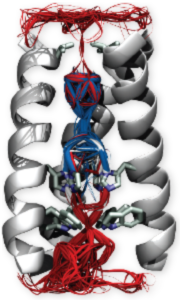
Voltage-gated cation channels
Voltage-gated cation channels are ubiquitous transmembrane proteins whose primary role is to transport selectively ions in a manner that depends on the polarization state of the membrane. They are involved in a variety of biological processes, among which the initiation and propagation of the nervous impulse. Mutations in genes encoding them are involved in a large number of inherited diseases (long QT syndrome, epilepsies, skeletal muscle paralyses, diabetes, deafness...). Voltage gated cation channels are composed of two main domains: the pore (including the gate, which opens and closes to let ions through or not, and the selectivity filter, which selects a specific type of ions) and the voltage sensor domain, which converts the electrical stimulus (change in the membrane potential) in a mechanical response (opening and closing of the channel).
Conduction Mechanism of Sodium
Selectivity is a fascinating propety of ion channels and can be attributed to the unique attributes of their selectivity filters. The resolution of the crystal structure of the first sodium selective channel has provided us with the first occasion to study the conduction and selectivity mechanisms in a such a selectivity filter. We use a combination of sophisticated simulation schemes (classical and QM/MM molecular dynamics simulation, free energy methods...) to compare these filters with the well-known potassium-selective ones.
Function and modulation of voltage-sensor domains
Voltage-sensor domains (VSD) are 4-helix transmembrane (TM) regulatory domains that undergo a conformational change in response to a change in the TM potential. Our work focuses on revealing the molecular-level fuctional mechanism of such domains and on providing an insight in their dysfunction due to genetic mutations.
References:
- L. Stock, L. Delemotte, V. Carnevale, W. Treptow and M. L. Klein, J. Phys. Chem. B, 117, 3782-3789 (2013) (Link).
- L. Delemotte, M. L. Klein, M. Tarek, Front. Pharmacol., 3, 97 (2012) (Link)
- C. Amaral, V. Carnevale, M. L. Klein and W. Treptow, Proc. Natl. Acad. Sci., 109, 21336-21341 (2012) (Link).
- P. Gosselin-Badaroudine, L. Delemotte, A. Moreau, M. L. Klein, and M. Chahine, Proc. Natl. Acad. Sci., 109, 19250-19255 (2012) (Link).
- W. Treptow, M. L. Klein, J. Phys. Chem. Lett., 3, 1017-1023 (2012) (Link).
- E. Vargas, V. Yarov-Yarovoy, F. Khalili-Araghi, W. A. Catterall, M. L. Klein, M. Tarek, E. Lindahl, K. Schulten, E. Perozo, F. Bezanilla and B. Roux, J. Gen. Physiol., 140, 587-594 (2012) (Link).
- L. Delemotte, M. Tarek, M. L. Klein, C. Amaral, and W. Treptow, Proc. Natl. Acad. Sci., 115, 6109-6114 (2011) (Link).
- V. Carnevale; W. Treptow; M. L. Klein, J. Phys. Chem. Lett., 2 (19), 2504-2508 (2011) (Link).
- L. Delemotte, W. Treptow, M. L. Klein, M. Tarek, Biophys. J., 99, L72-L74 (2010) (Link).
M2 Proton Channel of Influenza Virus A
The M2 protein from influenza virus A is a proton channel, responsible for the acidification of the interior of the virion. This process is required for the release of the genetic material into the host cell. Its inhibition constitutes one of the major strategies for treating influenza, and as such it is the target of two of the four available flu drugs. Unfortunately the recent emergence of drug-resistant strains has neutralized these two drugs. Despite the fact that the recent determination of the channel structure has greatly increased our understanding of the function of M2, much of molecular mechanism of proton transport and of drug-resistance remains to be elucidated.
Proton Conduction Mechanism
A full treatment of the dynamics of M2 is intrinsically challenging, however, because important events in the transport cycle occur on very different timescales and require different levels of description to model them accurately. Fast proton transfer processes are intrinsically coupled to much slower motions which are responsible for conformational changes in the protein. We adopt a combined approach involving experimental biophysics and theoretical investigations based on classical and hybrid quantum mechanics/molecular mechanics (QM/MM) molecular dynamics simulations. Our work elucidates the mechanism for proton transport in M2, where the protonation of specific pore-lining residues results in structural changes in the protein and pore waters.
Drug Binding
The design of effective anti-flu drugs requires a thorough understanding of the molecular mechanisms governing protein-drug binding and, more importantly, drug-resistance. The lack of structural information on the protein-drug complex, in particular for the S31N drug-resistant mutant, makes MD simulations a suitable approach to study the equilibrium configuration of channel-binding compounds. In order to capture the physico-chemical features impacting on the binding capabilities of a drug molecule, we use classical molecular dynamics simulations, complemented with approaches able to increase the sampling of rare events such as ABF and metadynamics, to screen libraries of compounds of known affinity.
References:
- J. Wang, C. Ma, J. Wang, H. Jo, B. Canturk, G. Fiorin, L. H. Pinto, R. A. Lamb, M. L. Klein and W. F. DeGrado, J. Med. Chem., 56, 2804-2812 (2013) (Link).
- A. Bankura, M. L. Klein and V. Carnevale, Chem. Phys., in press (2013) (Link).
- J. Wang, Y. Wu, C. Ma, G. Fiorin, J. Wang, L. H. Pinto, R. A. Lamb, M. L. Klein and W. F. DeGrado, Proc. Natl. Acad. Sci., 110, 1315-1320 (2013) (Link).
- J. Wang, C. Ma, G. Fiorin, V. Carnevale, T. Wang, F. Hu, R. A. Lamb, L. H. Pinto, M. Hong, M. L. Klein, W. F. DeGrado J. Am. Chem. Soc., 133(32), 12834-12841 (2011) (Link).
- E. Khurana, R. H. DeVane, M. Dal Peraro, M. L. Klein BBA-Biomembranes, 1808, 530-537 (2011) (Link).
- V. Balannik, V. Carnevale, G. Fiorin, B. G. Levine, R. A. Lamb, M. L. Klein, W. F. DeGrado, and L. H. Pinto, Biochemistry, 49, 696-708 (2010). (Link)
- V. Carnevale, G. Fiorin, B. G. Levine, W. F. DeGrado, and M. L. Klein, J. Phys. Chem. C, 114 (48), 20856-20863 (2010) (Link).
- R. Acharya, V. Carnevale, G. Fiorin, B. G. Levine, A. L. Polishchuk, V. Balannik, I. Samish, R. A. Lamb, L. H. Pinto, W. F. DeGrado, M. L. Klein, Proc. Natl. Acad. Sci. USA,107,15075 (2010) (Link).
- E. Khurana, M. Dal Peraro, R. DeVane, S. Vemparala, W.F. DeGrado, M.L. Klein, Proc. Natl. Acad. Sci. USA, 106, 1069-1074 (2009). (Link).
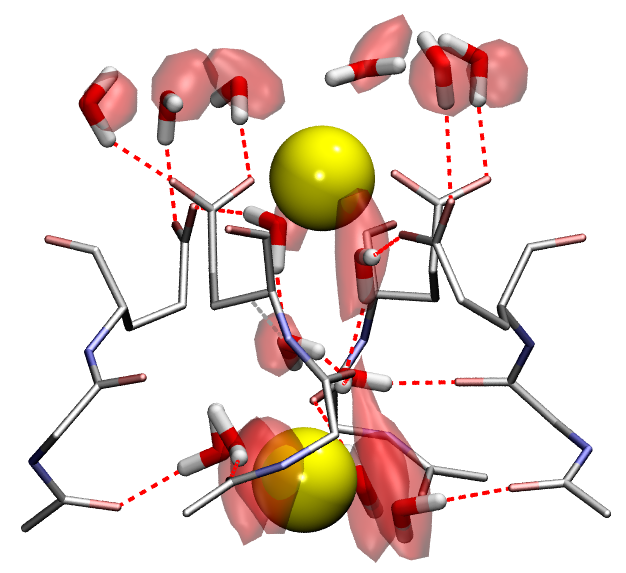
Metalloenzymes
Metalloproteins constitute between one third and half of the proteome of living organisms and around 40% of the proteins deposited in the Protein Data Bank contain metals. Among many essential structural and biological functions, they are crucial in respiration and photosynthesis, participate in DNA and RNA processing and are involved in pharmacologically important reactions, such as metabolism of xenobiotics, antibiotic resistance or defense against reactive oxygen species.
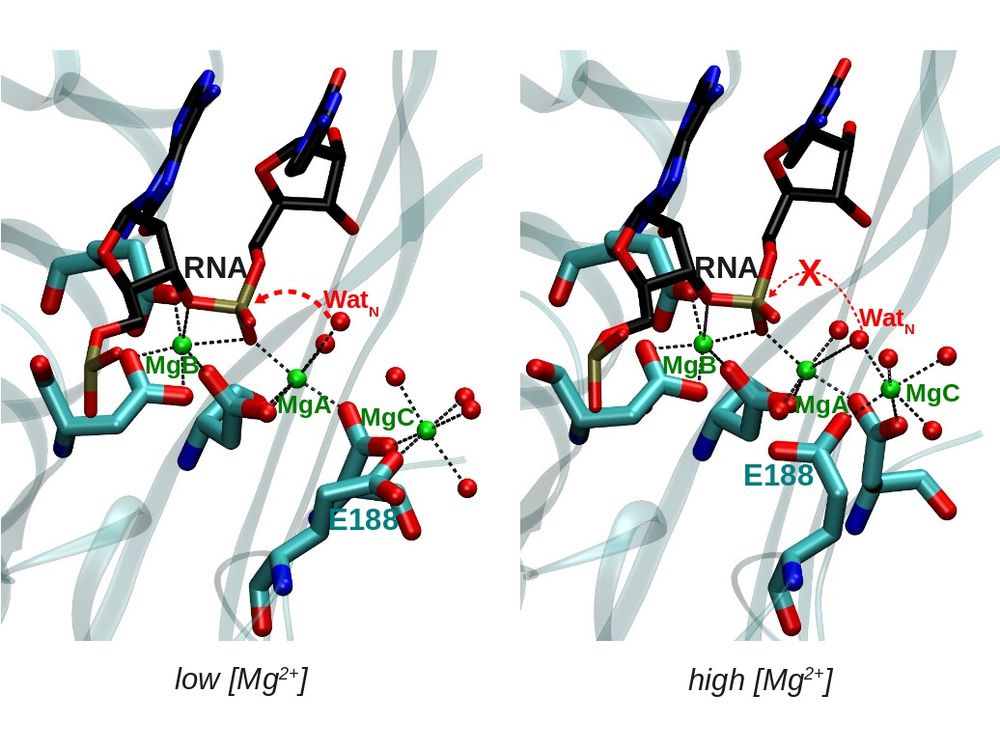
Endonucleases
Endonucleases are enzymes that cleave a phosphodiester bond within a RNA or DNA strand. The reaction is usually catalyzed by one or two divalent metal ions (in most cases Mg, but also Mn) bound to the enzyme. However, the number and nature of the metal ions involved in catalysis is not clear. Moreover, it is not well understood how the endonuclease activity is modulated by the metal concentration by post-translational modifications or by interaction with other proteins.
We use a combination of different computational methods (docking, classical molecular dynamics and QM and QM/MM calculations) in order to get further insight into the endonuclease activity of different ribonucleases.
This work is done in collaboration with the group of Prof. Allen W. Nicholson in the Department of Biology at Temple University.
References:
- S. Xiao, M. L. Klein, D. N. LeBard, B. G. Levine, H. Liang, C. M. MacDermaid and M. Alfonso-Prieto, J. Phys. Chem. B, 118, 873-889 (2014). (Link)
- M. Alfonso-Prieto and M. L. Klein. "Density Functional Theory-based treatments of metal binding sites in metalloenzymes: challenges and opportunities" in Metalloproteins: structure, function and interactions, CRC Press, in press (2014). (Link)
- M.H. Ho, M. De Vivo, M. Dal Peraro, and M. L. Klein, J. Am. Chem. Soc., 132, 13702-13712 (2010). (Link)
